For ECET 2026 aspirants, General Science—especially Chemistry—offers many easy-scoring opportunities. One high-weight topic is Chemical Bonding, often asked in multiple forms like bond types, electron sharing, and molecular shapes. This blog offers a quick, exam-oriented revision of the topic with 10 ECET-style MCQs, an answer key, and clear explanations to boost your Chemistry section scores.
📘 Concept Notes – Chemical Bonding
🧪 What is Chemical Bonding?
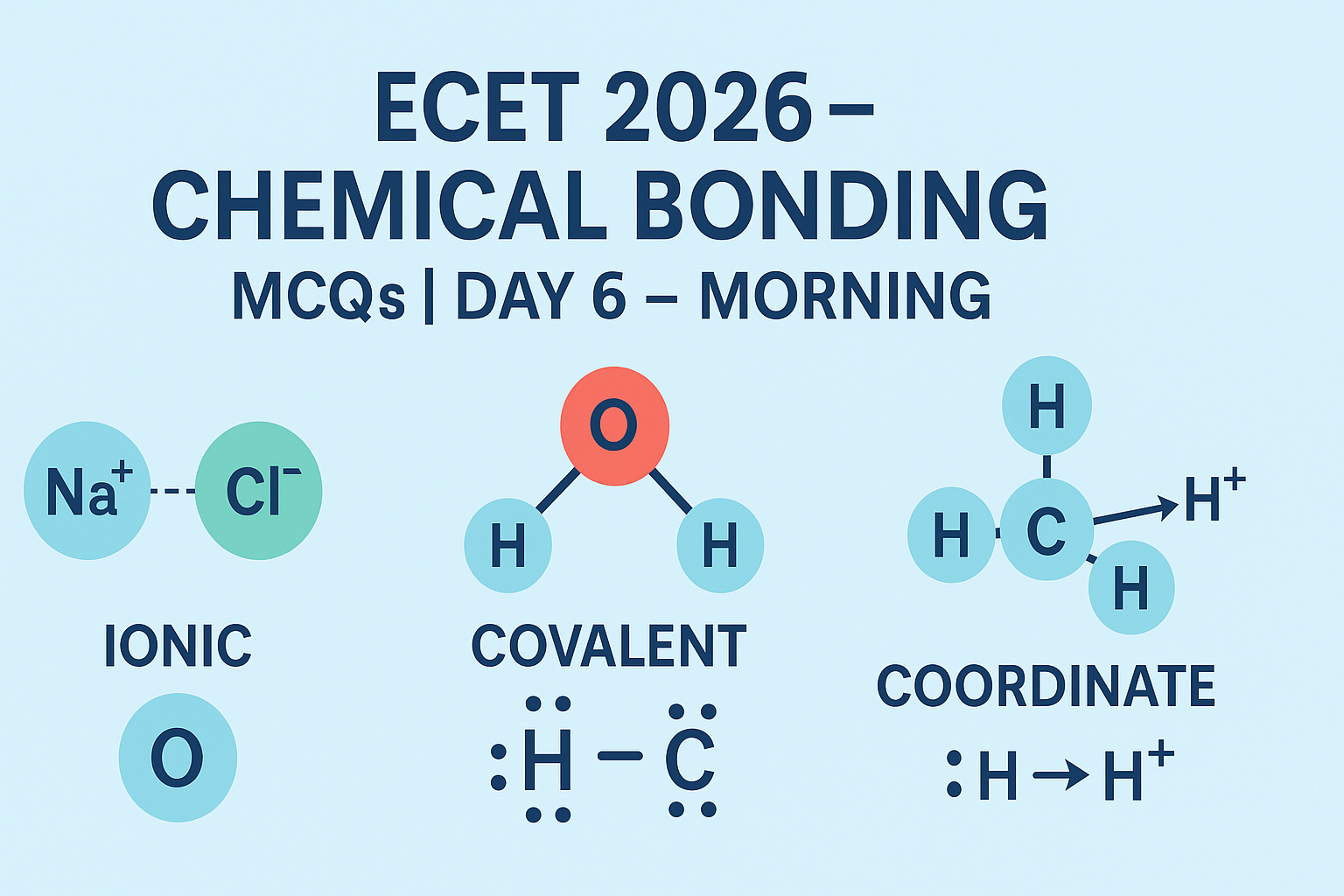
Chemical bonding is the force of attraction between atoms that enables the formation of compounds or molecules. Atoms bond to attain stability, often by achieving a full outer electron shell (octet rule).
🔷 Types of Chemical Bonds:
- Ionic Bond:
- Formed by transfer of electrons.
- One atom donates and another accepts electrons.
- Occurs between metals and non-metals.
- Example: NaCl (Sodium Chloride)
- Covalent Bond:
- Formed by sharing of electrons between atoms.
- Usually between non-metals.
- Example: H₂O, CO₂
- Coordinate Bond (Dative Bond):
- One atom donates both electrons for the shared pair.
- Found in complex ions like NH₄⁺.
- Metallic Bond:
- Involves delocalized electrons shared across a metal lattice.
- Gives metals their electrical conductivity and strength.
📌 Key Bonding Terms to Remember:
- Electronegativity: Atom’s tendency to attract shared electrons.
- Octet Rule: Atoms bond to complete 8 electrons in their outer shell.
- Bond Pair vs Lone Pair: Shared vs non-shared electron pairs.
- Valency: Number of electrons an atom can gain, lose, or share.
🔟 10 Most Expected MCQs – ECET 2026 [Chemical Bonding]
Q1. Ionic bonds are formed between:
A) Two metals
B) Two non-metals
C) Metal and non-metal
D) Noble gases
Q2. What type of bond is present in water (H₂O)?
A) Ionic
B) Covalent
C) Metallic
D) Coordinate
Q3. A bond formed by the transfer of electrons is:
A) Covalent
B) Ionic
C) Coordinate
D) Hydrogen
Q4. Which of the following is a covalent compound?
A) NaCl
B) MgCl₂
C) CH₄
D) CaO
Q5. The octet rule refers to:
A) Eight neutrons in a nucleus
B) Eight atoms in a molecule
C) Eight electrons in outer shell
D) Eight bonds in a molecule
Q6. Which bond involves a shared pair of electrons donated by only one atom?
A) Covalent
B) Ionic
C) Coordinate
D) Metallic
Q7. NaCl is an example of:
A) Covalent bond
B) Hydrogen bond
C) Coordinate bond
D) Ionic bond
Q8. Metallic bonds are present in:
A) NaCl
B) H₂
C) Cu
D) NH₃
Q9. Which of the following contains a coordinate bond?
A) CH₄
B) NH₄⁺
C) CO₂
D) O₂
Q10. Bond formed between hydrogen and oxygen in water is:
A) Ionic
B) Coordinate
C) Covalent
D) Metallic
✅ Answer Key Table
| Q.No | Answer |
|---|---|
| Q1 | C |
| Q2 | B |
| Q3 | B |
| Q4 | C |
| Q5 | C |
| Q6 | C |
| Q7 | D |
| Q8 | C |
| Q9 | B |
| Q10 | C |
🧠 Explanations of All Answers
- Q1 → C: Ionic bonds form between metals (electron donors) and non-metals (electron acceptors).
- Q2 → B: Water has polar covalent bonds between hydrogen and oxygen.
- Q3 → B: In ionic bonding, electrons are completely transferred.
- Q4 → C: CH₄ is a covalent compound formed by sharing electrons.
- Q5 → C: Octet rule means atoms aim for 8 electrons in their valence shell.
- Q6 → C: Coordinate bonds involve both electrons coming from one atom.
- Q7 → D: NaCl is formed by complete transfer of electrons (ionic).
- Q8 → C: Metallic bonding is a feature of metals like copper.
- Q9 → B: NH₄⁺ contains a coordinate bond donated by nitrogen.
- Q10 → C: Bonds in H₂O are covalent due to electron sharing.
🎯 Why This Practice Matters for ECET 2026
Chemical Bonding is one of the most frequently tested topics in ECET General Science. These questions are direct, concept-based, and easy to score. If you revise definitions, bond types, and examples, you can confidently secure marks in this section. This builds your science score, which contributes directly to overall ECET rank.
📲 Join Our ECET Prep Community on Telegram
Get access to daily quizzes, notes, PDFs, and expert tips on Chemistry and other ECET topics.
👉 Join us at: @LearnNewThingsHub