In ECET 2026 Chemistry, Fuels and Calorific Value is a high-weightage topic. Direct formula-based questions are asked every year. Understanding calorific value (CV) and comparing different fuels is crucial for solving numerical and theoretical problems.
📘 Concept Notes
🔥 What is Calorific Value (CV)?
- Definition: The amount of heat energy released when 1 kg of solid/liquid fuel or 1 m³ of gaseous fuel is completely burnt in oxygen.
- Unit:
 (for solid & liquid fuels),
(for solid & liquid fuels),  (for gaseous fuels).
(for gaseous fuels). - High CV → Better fuel efficiency.
⚙️ Types of Calorific Value
- Gross Calorific Value (GCV):
- Heat produced when fuel is burnt and water formed is condensed.
- Includes latent heat of condensation of water vapor.
![]()
Where:
 = mass of fuel burnt
= mass of fuel burnt = total heat produced
= total heat produced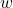 = weight of fuel
= weight of fuel
- Net Calorific Value (NCV):
- Heat produced when fuel is burnt but water vapor is not condensed (latent heat excluded).
![]()
Or in SI units:
![]()
Where:
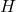 = mass fraction of hydrogen in the fuel
= mass fraction of hydrogen in the fuel
🔋 Comparison of Common Fuels
| Fuel | Approx. GCV (kJ/kg) | Remarks |
|---|---|---|
| Coal | 25,000 – 33,000 | Widely used, moderate efficiency |
| Petrol | ~ 47,000 | High calorific value, fast ignition |
| Diesel | ~ 45,000 | High CV, widely used in engines |
| LPG | ~ 46,000 | Clean burning, domestic fuel |
| Natural Gas | ~ 48,000 – 50,000 | Very high CV, eco-friendly |
| Hydrogen | ~ 1,20,000 | Highest CV, but storage is difficult |
📐 Example Problem
Q: A coal sample has: Carbon = 75%, Hydrogen = 5%, Oxygen = 8%, Sulphur = 1%, Nitrogen = 2%, Ash = 9%. Calculate GCV and NCV using Dulong’s formula.
Dulong’s Formula:
![]()
Substitute:
 ,
,  ,
,  ,
, 
![]()
![]()
![]()
Now,![]()
![]()
![]()
🔟 10 Expected MCQs – ECET 2026
Q1. Calorific value is defined as:
A) Heat required to raise 1 kg of water by 1°C
B) Heat produced by burning 1 kg/m³ of fuel completely
C) Heat content of 1 mole of fuel
D) Heat absorbed during combustion
Q2. Unit of calorific value for gaseous fuels is:
A) kJ/kg
B) kcal/mole
C) kJ/m³
D) kWh
Q3. Gross calorific value includes:
A) Latent heat of steam
B) Only sensible heat
C) Heat lost in flue gases
D) None
Q4. Net calorific value = ?
A) GCV + latent heat
B) GCV – latent heat
C) GCV × latent heat
D) GCV ÷ latent heat
Q5. Highest calorific value is for:
A) Diesel
B) Coal
C) Hydrogen
D) Petrol
Q6. In Dulong’s formula, oxygen reduces calorific value because:
A) It supports combustion
B) It combines with hydrogen
C) It forms ash
D) It is inert
Q7. Formula for NCV is:
A) ![]()
B) ![]()
C) ![]()
D) None
Q8. If GCV = 10,000 kcal/kg and H = 10%, then NCV = ?
A) 9000 kcal/kg
B) 9500 kcal/kg
C) 8500 kcal/kg
D) 8000 kcal/kg
Q9. Which gas fuel has maximum CV?
A) LPG
B) Natural Gas
C) Hydrogen
D) Coal gas
Q10. Dulong’s formula is used to calculate:
A) GCV of fuels
B) NCV of fuels
C) Ignition temperature
D) Flash point
✅ Answer Key
| Q.No | Answer |
|---|---|
| Q1 | B |
| Q2 | C |
| Q3 | A |
| Q4 | B |
| Q5 | C |
| Q6 | B |
| Q7 | B |
| Q8 | A |
| Q9 | C |
| Q10 | A |
🧠 Explanations
- Q1 → B: CV = heat produced by complete combustion of 1 kg/m³ of fuel.
- Q2 → C: For gases → kJ/m³.
- Q3 → A: GCV includes latent heat.
- Q4 → B: NCV = GCV – latent heat.
- Q5 → C: Hydrogen has highest calorific value.
- Q6 → B: Oxygen combines with hydrogen, reducing effective H content.
- Q7 → B: Correct formula.
- Q8 → A:
 → ~ 9000.
→ ~ 9000. - Q9 → C: Hydrogen > Natural Gas > LPG > Coal.
- Q10 → A: Dulong’s formula estimates GCV.
🎯 Why Practice Matters
- Questions on Calorific Value (CV) are direct formula-based.
- By practicing Dulong’s formula and NCV/GCV relation, students can secure 2–3 easy marks in Chemistry.
- Also useful in real-world applications like fuel selection, thermal efficiency, and energy management.