Concept Notes – Physics (Latent Heat in Real Time: Steam & Ice)
⚙️ Latent Heat
Latent heat is the amount of heat energy required to change the state of a substance without changing its temperature.
- Latent Heat of Fusion (Lf): Heat needed to convert 1 kg of solid into liquid at constant temperature.
- Latent Heat of Vaporization (Lv): Heat needed to convert 1 kg of liquid into vapor at constant temperature.
Formulas:
- Heat required for fusion:
![]()
Heat required for vaporization:
![]()
where,![]() = Heat (Joules)
= Heat (Joules)![]() = Mass (kg)
= Mass (kg)![]() = Latent heats
= Latent heats
🔬 Real-Time Examples
1️⃣ Ice to Water (Fusion):
When 1 kg of ice at ![]() melts into water at
melts into water at ![]() , it requires about 334,000 J of energy, but temperature does not rise.
, it requires about 334,000 J of energy, but temperature does not rise.
2️⃣ Water to Steam (Vaporization):
When 1 kg of water at ![]() converts into steam at
converts into steam at ![]() , it absorbs about 2,260,000 J of energy, with no temperature change.
, it absorbs about 2,260,000 J of energy, with no temperature change.
3️⃣ Daily Life Uses:
- Ice keeps drinks cool longer because it absorbs a large amount of heat while melting.
- Steam causes severe burns because it carries extra latent heat.
🔟 10 Most Expected MCQs – ECET 2026 [Physics]
Q1. Latent heat is the heat required to:
A) Raise temperature
B) Change state without temperature change
C) Cool substance
D) Increase pressure
Q2. Latent heat of fusion of ice is approximately:
A) 80 cal/g
B) 540 cal/g
C) 100 cal/g
D) 50 cal/g
Q3. Latent heat of vaporization of water is approximately:
A) 540 cal/g
B) 80 cal/g
C) 100 cal/g
D) 50 cal/g
Q4. During melting of ice, temperature:
A) Increases
B) Decreases
C) Remains constant
D) First decreases then increases
Q5. Heat required to convert 2 kg of ice at 0°C to water at 0°C is:![]()
A) 334 kJ
B) 668 kJ
C) 167 kJ
D) 500 kJ
Q6. Heat required to convert 1 kg water at 100°C to steam at 100°C is:![]()
A) 2260 kJ
B) 334 kJ
C) 1000 kJ
D) 540 kJ
Q7. The reason why steam causes severe burns compared to boiling water:
A) Higher temperature
B) Contains latent heat of vaporization
C) Low pressure
D) Less dense
Q8. Which statement is true?
A) Latent heat raises temperature
B) Latent heat changes state without temperature change
C) Latent heat reduces pressure
D) None
Q9. Which device uses latent heat principle?
A) Refrigerator
B) Fan
C) Battery
D) Transformer
Q10. Latent heat of fusion relates to:
A) Solid–liquid
B) Liquid–gas
C) Gas–plasma
D) Solid–plasma
✅ Answer Key
| Q.No | Answer |
|---|---|
| Q1 | B |
| Q2 | A |
| Q3 | A |
| Q4 | C |
| Q5 | B |
| Q6 | A |
| Q7 | B |
| Q8 | B |
| Q9 | A |
| Q10 | A |
🧠 Explanations of All Answers
- Q1 → B: Latent heat is used only to change the state, not temperature.
- Q2 → A: Ice → water = 80 cal/g (≈ 334 kJ/kg).
- Q3 → A: Water → steam = 540 cal/g (≈ 2260 kJ/kg).
- Q4 → C: Melting occurs at constant 0°C.
- Q5 → B:
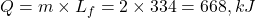 .
. - Q6 → A:
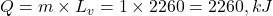 .
. - Q7 → B: Steam contains extra latent heat, hence burns are severe.
- Q8 → B: Correct definition of latent heat.
- Q9 → A: Refrigeration works on latent heat of vaporization.
- Q10 → A: Fusion = solid → liquid.
🎯 Why This Practice Matters for ECET 2026
- Latent Heat questions are direct, formula-based, and high scoring.
- At least 1–2 questions appear in every ECET Physics paper.
- Mastering formulas + real-life examples helps both exam & interviews.
📲 Join Our ECET Prep Community
👉 For daily MCQs, solved problems, and video lessons, join Telegram:
@LearnNewThingsHub