ECET 2026 lo Physics section lo Specific Heat & Calorimetry ane topic chala scoring. Questions ekkuga formulas-based untayi, so manchi practice chesina students ki easy marks vasthayi. I topic C-23 curriculum ki align ayyi, ECET exam lo repeated ga question bank lo kanipisthundi.
Concept Notes
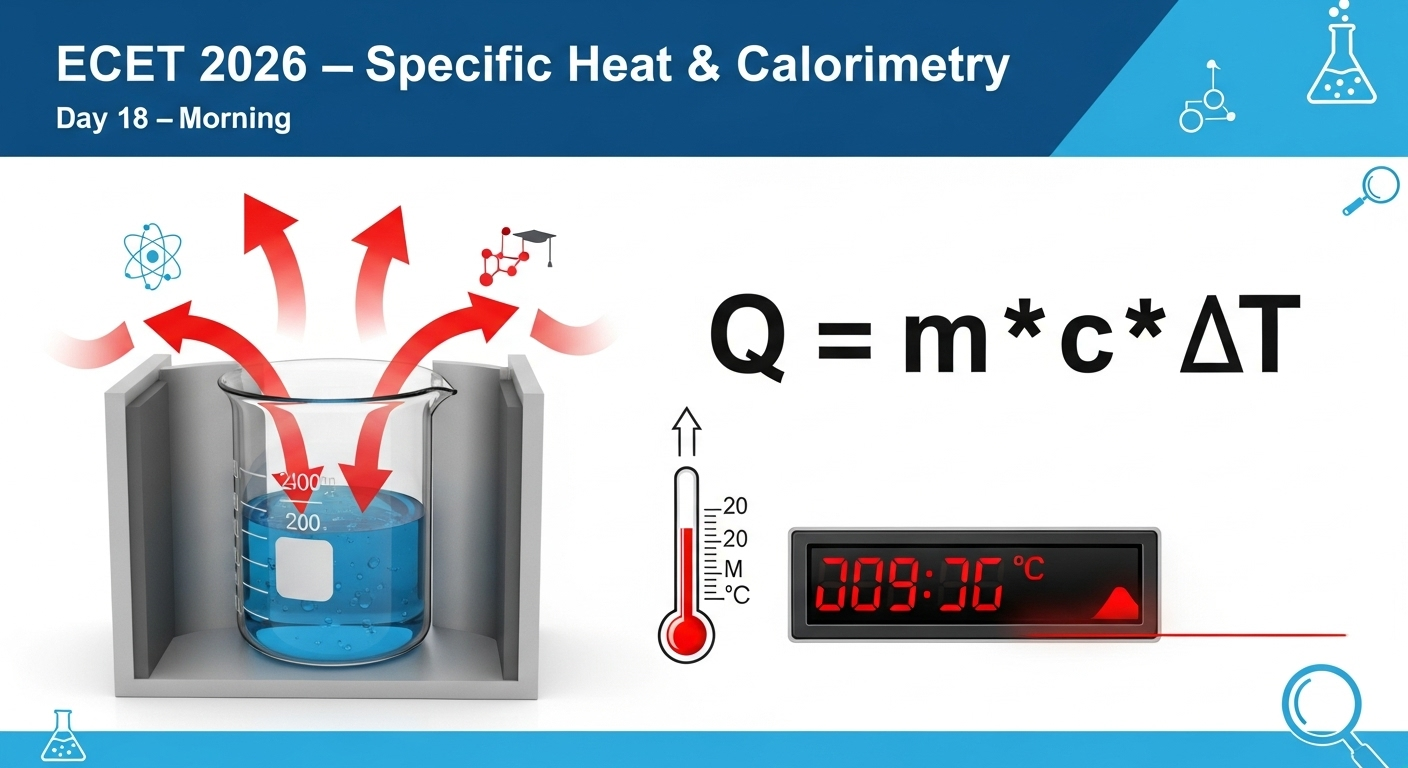
🌡️ Specific Heat Capacity
The specific heat capacity of a substance is the heat required to raise the temperature of 1 kg of that substance by 1°C (or 1 K).
Formula:
![]()
Where:
- QQQ = Heat supplied (Joules)
- mmm = Mass (kg)
- ccc = Specific heat capacity (J/kg\cdotpK)\text{(J/kg·K)}(J/kg\cdotpK)
- ΔT\Delta TΔT = Temperature change (K or °C)
Calorimetry
Calorimetry is the measurement of heat exchange between bodies using a calorimeter.
Principle of Calorimetry:
![]()
Where:
- m1,m2m_1, m_2m1,m2 = Masses of the substances
- c1,c2c_1, c_2c1,c2 = Specific heats
- T1,T2T_1, T_2T1,T2 = Initial temperatures
- TfT_fTf = Final equilibrium temperature
❄️ Latent Heat in Calorimetry
When there is a phase change, temperature does not change but heat is absorbed or released.
Formula:
![]()
Where:
- LLL = Latent heat (J/kg)
🔟 10 Most Expected ECET 2026 MCQs – Specific Heat & Calorimetry
Q1. The SI unit of specific heat capacity is:
A) J/kg·K
B) cal/g·°C
C) kJ/kg·K
D) Both A and B
Q2. If 2 kg of water is heated from 20°C to 40°C (c=4186 J/kg⋅K)(c = 4186 \, J/kg·K)(c=4186J/kg⋅K), the heat supplied is:
A) 167,440 J
B) 83,720 J
C) 125,000 J
D) 200,000 J
Q3. Calorimetry is based on which principle?
A) Conservation of mass
B) Conservation of heat
C) Conservation of energy
D) Conservation of momentum
Q4. Which of the following has the highest specific heat?
A) Water
B) Copper
C) Iron
D) Aluminium
Q5. Heat energy needed to change state without temperature change is called:
A) Sensible heat
B) Latent heat
C) Specific heat
D) Potential heat
Q6. In calorimetry, final temperature is reached when:
A) Heat lost = Heat gained
B) Mass is constant
C) Temperature is constant
D) No heat exchange occurs
Q7. 1 calorie = ? Joules
A) 4.18
B) 1.18
C) 2.18
D) 3.18
Q8. A calorimeter is used to measure:
A) Heat
B) Mass
C) Volume
D) Temperature
Q9. Specific heat depends on:
A) Nature of substance
B) Mass of substance
C) Volume of substance
D) Shape of substance
Q10. When ice melts into water, heat supplied is used for:
A) Increasing temperature
B) Breaking molecular bonds
C) Increasing mass
D) Increasing pressure
✅ Answer Key Table
| Q.No | Answer |
|---|---|
| Q1 | D |
| Q2 | A |
| Q3 | C |
| Q4 | A |
| Q5 | B |
| Q6 | A |
| Q7 | A |
| Q8 | A |
| Q9 | A |
| Q10 | B |
🧠 Explanations
- Q1 → D: Both J/kg·K (SI) and cal/g·°C are used in practice.
- Q2 → A:
![]()
![]()
- Q3 → C: Calorimetry uses the conservation of energy principle.
- Q4 → A: Water has the highest specific heat.
- Q5 → B: Latent heat is for phase changes without temperature change.
- Q6 → A: Thermal equilibrium is reached when heat lost = heat gained.
- Q7 → A: 1 calorie = 4.18 Joules.
- Q8 → A: Calorimeter measures heat exchange.
- Q9 → A: Depends only on the nature of the material.
- Q10 → B: Melting uses heat to break molecular bonds.
🎯 Why This Practice Matters
Specific Heat & Calorimetry questions are straightforward in ECET 2026. Most problems are direct formula substitutions. If you remember the formulas and units, you can score full marks in just a few seconds. This is one of the fastest-solving topics in Physics for CSE diploma students.
📲 Join Our ECET Prep Community
Get daily ECET MCQs, formula sheets, and practice papers in our Telegram group.
👉 @LearnNewThingsHub